PeriSorb® fully degradable peripheral vascular stent completes the first FIM trial enrollment
Release time:
2021-12-25 09:00
Source:
Recently, the clinical study of the fully degradable peripheral vascular drug-eluting stent system (PeriSorb®) with Professor Chen Zhong from Beijing Anzhen Hospital Affiliated to Capital Medical University as the coordinating investigator completed FIM (First in Man) in Beijing Friendship Hospital Affiliated to Capital Medical University. The first patient in the trial was enrolled.
On December 10, 2021, the PeriSorb® clinical project held an FIM trial investigator meeting under the auspices of Professor Chen Zhong, and officially launched the FIM clinical trial of the PeriSorb® stent. Subsequently, the project has successively completed their respective FIM test kick-off meetings in several centers.
On December 15, 2021, one of the participating units of the PeriSorb® stent FIM trial, director Chen Xueming and Feng Hai of Beijing Friendship Hospital affiliated to Capital Medical University, and their team successfully completed the first implantation of the PeriSorb® stent.

Figure 1 Group photo of Director Feng Hai's team after stent implantation
According to Director Feng Hai, the patient was a 57-year-old female. Angiography results showed that the middle segment of the left superficial femoral artery was narrowed by more than 90%. Director Feng Hai and his team successfully implanted a 5.0×76mm Ammet PeriSorb® stent for this patient. The entire operation went very smoothly. The patient's condition was stable after the operation, the blood supply to the lower extremities recovered well, and the pain symptoms of the lower extremities were significantly improved. One week after surgery, the patient's Rutherford classification improved from preoperative grade 3 to grade 1, and the ankle-brachial index (ABI) recovered from preoperative 0.75 to 1.05. The patient has been successfully discharged from the hospital.
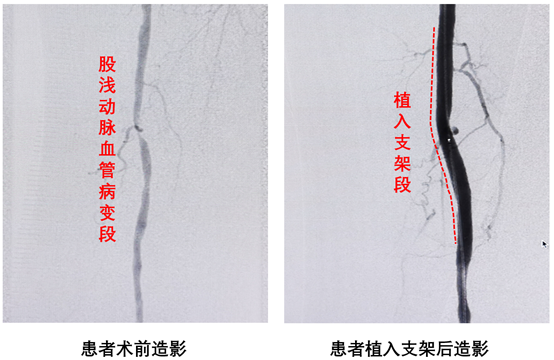

Figure 2 Group photo of the patient with the surgical team after surgery
This is the world's first 3D printed fully degradable peripheral stent implanted in the human body, marking that my country's 3D printing fully degradable peripheral stent with completely independent intellectual property rights has taken the lead in entering the clinical trial stage, and it also marks that my country's 3D printing technology has advanced to the world's leading level. A big step.
Peripheral arterial disease (PAD) of the lower extremities has attracted great attention due to its high prevalence and high incidence of cardiovascular and cerebrovascular events. PAD often causes discomfort or pain when walking, which can occur in various parts of the lower extremities. In severe cases, if the vascular stenosis rate exceeds 75%, persistent pain in the feet or toes will occur, and further extremity ulcers will occur. If local circulation is not improved, these ulcers can dry out and turn black and eventually die.
In recent years, with the increasing aging of the population in my country and the change of lifestyle, the incidence of PAD has risen sharply. A study published in 2019 on the prevalence of peripheral arterial disease (PAD) in people aged ≥35 years in Beijing showed that the prevalence of PAD in people aged ≥35 years in Beijing was 3.84%, and the prevalence of PAD in people aged 60 years was 3.84%. 6.34%, the prevalence of PAD increased sharply with age. Based on this, it is estimated that the number of PAD patients in the country may have reached more than 50 million.
Peripheral arterial disease not only seriously affects the quality of life of patients, but also causes a huge social and economic burden, and has become a major public health medical and social problem that needs to be solved urgently in my country.
Interventional therapy has become the first choice for the treatment of peripheral vascular disease because of its small trauma, quick effect, quick recovery, and low risk of restenosis.
The main devices currently used in interventional therapy include: ordinary balloons, metal stents, drug balloons, etc. Fully degradable peripheral vascular drug-eluting stents have attracted great attention due to their advantages of preventing acute retraction of vascular lesions, local and slow drug release, and complete degradation. However, after the peripheral stent is implanted in the body, it will withstand complex stresses such as bending, stretching, squeezing and torsion repeatedly exerted by the muscles during human activities, which makes the stent prone to fatigue and fracture. Therefore, the development of fully degradable peripheral vascular stents is extremely challenging. At present, there is no fully degradable peripheral vascular stent that has been popularized and applied clinically.
Amet has adopted a number of patented technologies in the development of PeriSorb® fully degradable stents, such as 3D multi-axis precision printing technology, ultra-compliant closed-loop stent structure design, single-sided targeted paclitaxel drug coating technology and new fatigue-resistant fully degradable materials , thereby greatly improving the radial support force and fatigue resistance of the fully degradable stent, and the PeriSorb® stent is expected to become the preferred device for the interventional treatment of peripheral artery disease.
About Beijing Amet: Beijing Amet Medical Equipment Co., Ltd. is a high-tech enterprise in Zhongguancun, the first batch of "Biopharmaceutical Industry Leapfrog Development Project" (G20) enterprises in Beijing's "Thirteenth Five-Year Plan" period, and a specialized, specialized, and new enterprise in Beijing . The 3D multi-axis precision printing and manufacturing technology for the preparation of fully degradable vascular stents owned by Amet is an internationally pioneered vascular stent additive manufacturing technology. At present, 12 invention patents have been authorized in China, the United States and the European Union.
Another innovative product of Amet, the fully degradable coronary stent AMSorb® stent, has entered the special review channel for innovative medical devices of the State Food and Drug Administration and is currently undergoing large-scale registration clinical trials. Amet's fully degradable vascular stent has successively won the "Twelfth Five-Year" Support Program Project of the Ministry of Science and Technology, the "Thirteenth Five-Year" Major R&D Project, the National Natural Science Foundation of China, the Beijing Municipal Science and Technology Commission, and the Zhongguancun Administrative Committee during the research and development process. , Haidian District and Daxing District Science and Technology Commission and other scientific research projects.
Previous Page


